Good News Update!
24 SEPTEMBER 2021 • Ontario government approves coverage for Trikafta in people with CF, who are age 12 or older and have at least one copy of the F508del (also known as deltaF508 or “delta F”) mutation. At the moment, only people with FEV1 of < 90% predicted will be eligible for Trikafta.
This is wonderful news and an incredibly fast response from the government. From Health Canada approval to agreement by the province to cover has occurred in only 3 months! This is a testament to the advocacy work done by the CF community.
Below is an explanation of what still needs to happen before you can actually start taking Trikafta. After reading this latest information, please see the additional FAQs further down the page for more information about the drug itself and its affect on the body. Contact cftrmodulatorinfo@unityhealth.to with questions.
How do people get Trikafta covered through the provincial drug plans?
In Ontario, the provincial drug plan is called the Ontario Drug Benefit (ODB) program. To qualify for ODB you must be a citizen in Ontario and fit in any of the following categories:
- be 65 years of age or older
- 24 years of age or younger and not covered by a private insurance plan (OHIP Plus)
- receiving professional home services (Home Care)
- receiving benefits from Ontario Works or the Ontario Disability Support Program
- be enrolled in the Trillium Drug Program
For more details about how to access the ODB Program, see https://www.ontario.ca/page/check-medication-coverage/
What is the Trillium Drug Program?
The Trillium drug program (TDP) offers financial assistance for Ontario residents who have high prescription drug costs in relation to their net household income. This plan is based on coverage of the cost of medications by the government after you pay a deductible that is based on a portion (~4%) of your net household income.
You qualify for the TDP if you:
- Have valid Ontario Health Insurance (OHIP) and are a resident of Ontario.
- Are not eligible for drug coverage as another category of recipient under the Ontario Drug Benefit (ODB) Program (see above).
- Do not have private insurance coverage or your private insurance does not cover 100% of your prescription drug costs.
To apply for TDP you must download and complete an application form.
You will need the information from your income tax return for the prior year as well as the tax return information for other adults in your household.
The completed application is sent or emailed to trillium@ontariodrugbenefit.ca or faxed (416 642 3034) to the Ministry of Health. It takes about 15 business days (3 weeks) for your application to be reviewed and for you to receive a response with information about the amount of your deductible.
I already have Trillium coverage (or have government drug coverage through OW/ODSP), can I get a prescription for Trikafta?
Unfortunately, there is still another step needed.
Trikafta is an expensive medication and is only eligible for patients with CF and the F508del mutation. Due to these specialized criteria, to access Trikafta through government funding, an application to the Exceptional Access Program (EAP) must be made.
This requires the CF clinic to submit a request, in writing, to the EAP documenting relevant medical information. This includes providing the clinical rationale for use of Trikafta, proof that you have the appropriate genetic mutations as well as baseline clinical information such as:
- lung function within the last 3 months,
- number of chest infections and hospital admissions in the last 6 months,
- weight, height and body mass index and
- measurement of quality of life through completion of a questionnaire.
All requests are reviewed according to specific guidelines and criteria. If EAP approval is granted, the coverage period begins as of the effective date and extends only to the specified date. CADTH recommended that assessment of initial response should occur after 6 months of starting on Trikafta and annually thereafter. The Exceptional Access Program receives between 250 and 500 applications a day. Applications are categorized in order of priority – how quickly a drug is needed, the type of drug, and the condition for which the drug is being used. We do not know what the turnaround time for Trikafta will be.
What do I do if my FEV1 is above 90% predicted?
Unfortunately, CADTH recommended that Trikafta only be covered in people with CF whose FEV1 is below 90% predicted. We do not agree with this limitation as one purpose of CFTR modulator therapy is to prevent lung damage rather than waiting until damage is present before treating. There is also evidence from the studies of Trikafta in children that there can still be improvement in lung function even in those people whose lung function is above 90% predicted when they start therapy. It appears that the EAP has applied this limitation in determining who can access Trikafta although it is not clear what the private insurance providers will do. For our patients who will access Trikafta through EAP, we will still apply for access even if your lung function is above 90% predicted.
What are next steps if I have private insurance?
Vertex has set up a patient assistance program, called Village, which will help assess your private insurance coverage for Trikafta.
Since July we have been working to enrol all of our patients with private insurance on the Village patient assistance program. As part of the enrollment process you will have to provide consent for your contact information, insurance status and some limited information about your diagnosis with the program. If you have access to private drug benefits, please email cftrmodulatorinfo@unityhealth.to to get the enrollment form.
Once you are enrolled, the assistance program will contact your insurance provider(s) and determine if Trikafta is covered by your plan as well as any coverage limits or additional information required from your healthcare team. The assistance program may also assess your financial situation in order to provide limited financial assistance if your insurance does not provide full coverage.
Once it is established whether you are covered for Trikafta or not, the Village patient assistance program will contact us to advise about next steps (i.e. what medical information and paperwork needs to be provided by the clinic for prior authorization).
I have private insurance but my plan is not yet covering Trikafta. Why is there a delay?
Trikafta was only approved by Health Canada on June 23, 2021 and it can take some time for the insurance companies to review a new medication and decide if they will add that medication to their plan. The insurance companies may wait to hear what the recommendations are from CADTH before deciding what medications they will cover. The CADTH final recommendation on Trikafta was just published on September 16th. Now that Ontario, Alberta and Saskatchewan have agreed to follow the CADTH recommendation that Trikafta should be covered by provincial plans, we anticipate that the insurance companies will make their decision on coverage of Trikafta soon.
I have private insurance and I have been informed that my insurer will cover Trikafta. What happens now?
The CF clinic will be asked to complete a prior authorization form which will be reviewed to confirm that you meet the criteria set by the insurer. This information may include your genetic mutations, lung function, number of chest infections and hospital admissions in the last 6 months, weight, height and body mass index and measurement of quality of life, and sweat test results.
Can I access Trikafta through the Trillium Drug Program if I have private insurance?
Yes, if you have a private insurance plan but it does not cover Trikafta you can still apply for Trillium but there is additional information needed on your application. You must provide information about your private insurance and provide a letter from your insurance company to say when your insurance started and ended (if applicable), the date that you reached your annual or lifetime maximum, the name of the drug that is not covered by your plan and the amount of the premium you pay.
How will my insurance company or the government insurance through EAP decide to extend coverage for Trikafta?
The EAP and private insurers will have criteria for renewal based on demonstration of clinical improvement. CADTH has recommended assessment after 6 months of Trikafta to see if it is working and to determine if coverage will continue. The EAP will most likely follow this recommendation and the private plans probably will as well. Thereafter reassessment will be annually.
The reassessment will need to be done at about 4 months after starting Trikafta in order to get all the measurements done, to complete the paperwork and to allow time for the EAP program or the private plan to review the data and make a decision. The EAP suggests renewals be submitted 6 weeks before coverage ends.
These measurements may include lung function, rates of lung infections, hospital admissions, weight, sweat test, measurements of quality of life, and evidence of decreased lung damage (CT scan).
CADTH has stated that approval to continue therapy will require one of the following to demonstrate benefit:
- Improvement in FEV1 of 5% predicted from a baseline lung function done within 3 months of starting Trikafta.
- Reduction in the number of hospitalizations in the 6 months since starting Trikafta compared to the 6 months before Trikafta.
- Reduction in the number of days of oral or IV antibiotics in the 6 months since starting Trikafta compared to the 6 months before Trikafta.
- Reduction in the total number of pulmonary exacerbations requiring oral or IV antibiotics in the 6 months since starting Trikafta compared to the 6 months before Trikafta.
- No decline in weight compared with baseline within 3 months of starting Trikafta
- Improvement by 4 points or more in the CFQ-R quality of life test.
For this reason, we will be doing baseline testing of all these measures to make sure we can demonstrate any benefits of Trikafta and ensure ongoing coverage.
What is the Toronto Adult CF Clinic doing to help eligible patients get access to Trikafta?
- The Toronto Adult CF Clinic has a database that has allowed us to identify all of our patients with CF that have at least 1 copy of the F508del mutation and thus would be eligible for Trikafta therapy.
- We also have the ability to identify those patients who have private insurance.
- We are receiving direct updates about which insurance plans have agreed to cover Trikafta.
- We are enrolling patients in the patient assistance program (Village).
- We are increasing our staffing of pharmacists and drug access navigators to help in the enrollment process.
- We have set up sweat testing facilities at St Michael’s Hospital (sweat tests needed prior to starting therapy and for follow up) so that sweat testing can be done during your regular CF clinic visit (starting October 19th)
- We will be completing the paperwork needed by private insurers (third party payers) to approve coverage (i.e. prior authorization forms).
- We will be completing the paperwork needed by the government to approve coverage (i.e. Exceptional Access Program).
There are a lot of people with CF eligible for Trikafta and access to therapy is a time-consuming process. How will the CF clinic prioritize the order in which patients are enrolled?
There are over 375 patients in our clinic eligible for Trikafta. That is a lot of applications!
We have identified the people with CF eligible for Trikafta based on their CF mutations and have created a spreadsheet which includes lung function (FEV1) and whether the person has private or government funding.
We have decided that we will process applications – whether through EAP (government funding) or prior authorization (private insurance) – based on severity of disease as measured by lung function. This means that we will be starting with those patients in our clinic who have the lowest lung function.
In Ontario, the provincial drug plan is called the Ontario Drug Benefit (ODB) program. To qualify for ODB you must be a citizen in Ontario and fit in any of the following categories:
- be 65 years of age or older
- 24 years of age or younger and not covered by a private insurance plan (OHIP Plus)
- receiving professional home services (Home Care)
- receiving benefits from Ontario Works or the Ontario Disability Support Program
- be enrolled in the Trillium Drug Program
For more details about how to access the ODB Program, see https://www.ontario.ca/page/check-medication-coverage/
The Trillium drug program (TDP) offers financial assistance for Ontario residents who have high prescription drug costs in relation to their net household income. This plan is based on coverage of the cost of medications by the government after you pay a deductible that is based on a portion (~4%) of your net household income.
You qualify for the TDP if you:
- Have valid Ontario Health Insurance (OHIP) and are a resident of Ontario.
- Are not eligible for drug coverage as another category of recipient under the Ontario Drug Benefit (ODB) Program (see above).
- Do not have private insurance coverage or your private insurance does not cover 100% of your prescription drug costs.
To apply for TDP you must download and complete an application form.
You will need the information from your income tax return for the prior year as well as the tax return information for other adults in your household.
The completed application is sent or emailed to trillium@ontariodrugbenefit.ca or faxed (416 642 3034) to the Ministry of Health. It takes about 15 business days (3 weeks) for your application to be reviewed and for you to receive a response with information about the amount of your deductible.
Unfortunately, there is still another step needed.
Trikafta is an expensive medication and is only eligible for patients with CF and the F508del mutation. Due to these specialized criteria, to access Trikafta through government funding, an application to the Exceptional Access Program (EAP) must be made.
This requires the CF clinic to submit a request, in writing, to the EAP documenting relevant medical information. This includes providing the clinical rationale for use of Trikafta, proof that you have the appropriate genetic mutations as well as baseline clinical information such as:
- lung function within the last 3 months,
- number of chest infections and hospital admissions in the last 6 months,
- weight, height and body mass index and
- measurement of quality of life through completion of a questionnaire.
All requests are reviewed according to specific guidelines and criteria. If EAP approval is granted, the coverage period begins as of the effective date and extends only to the specified date. CADTH recommended that assessment of initial response should occur after 6 months of starting on Trikafta and annually thereafter. The Exceptional Access Program receives between 250 and 500 applications a day. Applications are categorized in order of priority – how quickly a drug is needed, the type of drug, and the condition for which the drug is being used. We do not know what the turnaround time for Trikafta will be.
Since July we have been working to enrol all of our patients with private insurance on the Village patient assistance program. As part of the enrollment process you will have to provide consent for your contact information, insurance status and some limited information about your diagnosis with the program. If you have access to private drug benefits, please email cftrmodulatorinfo@unityhealth.to to get the enrollment form.
Once you are enrolled, the assistance program will contact your insurance provider(s) and determine if Trikafta is covered by your plan as well as any coverage limits or additional information required from your healthcare team. The assistance program may also assess your financial situation in order to provide limited financial assistance if your insurance does not provide full coverage.
Once it is established whether you are covered for Trikafta or not, the Village patient assistance program will contact us to advise about next steps (i.e. what medical information and paperwork needs to be provided by the clinic for prior authorization).
Yes, if you have a private insurance plan but it does not cover Trikafta you can still apply for Trillium but there is additional information needed on your application. You must provide information about your private insurance and provide a letter from your insurance company to say when your insurance started and ended (if applicable), the date that you reached your annual or lifetime maximum, the name of the drug that is not covered by your plan and the amount of the premium you pay.
The EAP and private insurers will have criteria for renewal based on demonstration of clinical improvement. CADTH has recommended assessment after 6 months of Trikafta to see if it is working and to determine if coverage will continue. The EAP will most likely follow this recommendation and the private plans probably will as well. Thereafter reassessment will be annually.
The reassessment will need to be done at about 4 months after starting Trikafta in order to get all the measurements done, to complete the paperwork and to allow time for the EAP program or the private plan to review the data and make a decision. The EAP suggests renewals be submitted 6 weeks before coverage ends.
These measurements may include lung function, rates of lung infections, hospital admissions, weight, sweat test, measurements of quality of life, and evidence of decreased lung damage (CT scan).
CADTH has stated that approval to continue therapy will require one of the following to demonstrate benefit:
- Improvement in FEV1 of 5% predicted from a baseline lung function done within 3 months of starting Trikafta.
- Reduction in the number of hospitalizations in the 6 months since starting Trikafta compared to the 6 months before Trikafta.
- Reduction in the number of days of oral or IV antibiotics in the 6 months since starting Trikafta compared to the 6 months before Trikafta.
- Reduction in the total number of pulmonary exacerbations requiring oral or IV antibiotics in the 6 months since starting Trikafta compared to the 6 months before Trikafta.
- No decline in weight compared with baseline within 3 months of starting Trikafta
- Improvement by 4 points or more in the CFQ-R quality of life test.
For this reason, we will be doing baseline testing of all these measures to make sure we can demonstrate any benefits of Trikafta and ensure ongoing coverage.
- The Toronto Adult CF Clinic has a database that has allowed us to identify all of our patients with CF that have at least 1 copy of the F508del mutation and thus would be eligible for Trikafta therapy.
- We also have the ability to identify those patients who have private insurance.
- We are receiving direct updates about which insurance plans have agreed to cover Trikafta.
- We are enrolling patients in the patient assistance program (Village).
- We are increasing our staffing of pharmacists and drug access navigators to help in the enrollment process.
- We have set up sweat testing facilities at St Michael’s Hospital (sweat tests needed prior to starting therapy and for follow up) so that sweat testing can be done during your regular CF clinic visit (starting October 19th)
- We will be completing the paperwork needed by private insurers (third party payers) to approve coverage (i.e. prior authorization forms).
- We will be completing the paperwork needed by the government to approve coverage (i.e. Exceptional Access Program).
We have identified the people with CF eligible for Trikafta based on their CF mutations and have created a spreadsheet which includes lung function (FEV1) and whether the person has private or government funding.
We have decided that we will process applications – whether through EAP (government funding) or prior authorization (private insurance) – based on severity of disease as measured by lung function. This means that we will be starting with those patients in our clinic who have the lowest lung function.
FAQs about Trikafta
Your CF Care team has put together this list of frequently asked questions. If you have questions after reading this document, please feel free to reach us at cftrmodulatorinfo@unityhealth.to.
What is Trikafta
Trikafta is also known as triple-combination therapy as it is a medication made up of three different modulators — tezacaftor/ivacaftor (which make up Symdeko) combined with elexacaftor.
Trikafta is an oral medication (i.e. a pill) that is taken twice a day. Trikafta must be used daily to continue to help the CFTR protein work effectively.
Is Trikafta Gene Therapy?
No, Trikafta does not act on the genetic material in the body, only on the CFTR protein.
What are CFTR Modular Drugs?
CFTR modulators are a new type of medication that work directly on the defective CFTR protein to facilitate the movement of chloride and bicarbonate and thus correct the problem with the excessively thick mucus. The type of CFTR modulator medication must be matched to the specific mutations seen in an individual with CF.
Ivacaftor (Kalydeco™) was the first modulator that was commercially available. Ivacaftor is designed to fix CFTR function in people with CF who have the G551D mutation.
What are some examples of CFTR that are commercially available?
Some mutations require a combination of 2 modulators together in 1 pill.
- Orkambi is a combination of ivacaftor and lumacaftor.
- Symdeko is a combination of ivacaftor and tezacaftor.
- Both Orkambi and Symdeko are designed to fix CFTR function in people with CF who have 2 copies of the deltaF508 (also known as F508del) mutation.
Who is eligible to take Trikafta?
Trikafta works in people with CF who have at least one copy of the deltaF508 mutation, regardless of their second mutation. Trikafta is currently approved for people that are age 12 years or older although studies have been completed in people with CF between the ages of 6 and 12 years.
How can I find out about my CF mutations?
- You can ask your CF physician at your clinic visit.
- You can look up your CF mutation on the CF Canada Patient Registry. You can be registered to have access to this patient database at your CF clinic visit or by contacting cftrmodulatorinfo@unityhealth.to
- If you have provided email consent, we can email you the names of your CF mutations and if you are eligible for Trikafta. Please e-mail us at cftrmodulatorinfo@unityhealth.to
Trikafta is also known as triple-combination therapy as it is a medication made up of three different modulators — tezacaftor/ivacaftor (which make up Symdeko) combined with elexacaftor.
Trikafta is an oral medication (i.e. a pill) that is taken twice a day. Trikafta must be used daily to continue to help the CFTR protein work effectively.
No, Trikafta does not act on the genetic material in the body, only on the CFTR protein.
CFTR modulators are a new type of medication that work directly on the defective CFTR protein to facilitate the movement of chloride and bicarbonate and thus correct the problem with the excessively thick mucus. The type of CFTR modulator medication must be matched to the specific mutations seen in an individual with CF.
Ivacaftor (Kalydeco) was the first modulator that was commercially available. Ivacaftor is designed to fix CFTR function in people with CF who have the G551D mutation.
Some mutations require a combination of 2 modulators together in 1 pill.
- Orkambi is a combination of ivacaftor and lumacaftor.
- Symdeko is a combination of ivacaftor and tezacaftor.
- Both Orkambi and Symdeko are designed to fix CFTR function in people with CF who have 2 copies of the deltaF508 (also known as F508del) mutation.
Trikafta works in people with CF who have at least one copy of the deltaF508 mutation, regardless of their second mutation. Trikafta is currently approved for people that are age 12 years or older although studies have been completed in people with CF between the ages of 6 and 12 years.
- You can ask your CF physician at your clinic visit.
- You can look up your CF mutation on the CF Canada Patient Registry. You can be registered to have access to this patient database at your CF clinic visit or by contacting cftrmodulatorinfo@unityhealth.to
- If you have provided email consent, we can email you the names of your CF mutations and if you are eligible for Trikafta. Please e-mail us at cftrmodulatorinfo@unityhealth.to
Trikafta works in people with CF who have at least one copy of the deltaF508 mutation, regardless of their second mutation.
Trikafta & Your Body
When will Trikafta be available in Canada?
Trikafta was approved by Health Canada on June 18, 2021. This means that the medication has received a Drug Identification Number (DIN), has met the standards of the Foods and Drugs Act and can be prescribed in Canada.
However, Trikafta™ now has to be produced for sale in Canada under this new DIN and thus Trikafta™ will not be available for sale until the end of July 2021.
What are the steps to determine who can get Trikafta?
After a new drug is approved by Health Canada, there are different ways that the medication can be accessed:
- Covered under private (3rd party) insurance plans. For example
- Covered under the provincial drug plans (i.e. Trillium program, ODSP, ODB) and
- Directly paid for by the individual.
How much does Trikafta cost?
The list price of Trikafta in the USA is USD $311,000 per year. We do not know what the actual cost of the medicine will be for the private and government payers.
Will private insurance cover Trikafta?
Private insurers perform their own internal review of new medications to decide if they will add them to their list of medications that they will cover. This process takes about 1-3 months but can be longer.
Not all insurance companies will cover CFTR modulators. Even if an insurance company covers CFTR modulators, not all drug plans with that insurance company will include CFTR modulators.
What are next steps if I have private insurance?
Vertex has set up a patient assist program (Village) which will help assess your private insurance coverage for Trikafta™.
After a discussion at your clinic visit or on the phone, you will be provided with an enrollment form for the Village patient assistance program which you will need to complete in order to access the program. As part of the enrollment process you will have to provide consent for your contact information, insurance status and some limited information about your diagnosis to be shared with the program.
Once you are enrolled, the assistance program will contact your insurance provider(s) and determine if Trikafta™ is covered by your plan as well as any coverage limits or additional information required from your healthcare team. The assistance program may also assess your financial situation in order to provide limited financial assistance if your insurance does not provide full coverage.
Once it is established whether you are covered for Trikafta™ or not, the Village patient assistance program will contact us to advise about next steps (i.e. what medical information and paperwork needs to be provided by the clinic for prior approval).
What happens to people who are receiving Trikafta™ though the Special Access compassionate program?
The Special Access Program was importing Trikafta into Canada when it was not Health Canada approved. Now that Health Canada has approved Trikafta, everyone on the Special Access compassionate program must be moved on to the Compassionate program with Health Canada-approved medication. We will be contacting all of those people on the Compassionate program to have them enrol in the Village Patient Assistance Program to facilitate this switch. Coverage through the Compassionate Program will continue until Trikafta is available either through the individual’s private insurance or through the provincial formulary.
What is the process to determine if the provincial drug plan will cover Trikafta?
After Health Canada approval, a committee called CADTH (Canadian Agency for Drugs and Technologies in Health) reviews new medications to make recommendations if they should be covered by the drug plan provided by each province. We are awaiting CADTH’s decision about Trikafta™ and this is expected in July 2021.
If CADTH recommends that Trikafta should be on the list of medications paid for by the province (i.e. the provincial formulary), then the next step is for the government to negotiate how much they will pay for Trikafta. This is done by a committee called pCPA (pan-Canadian Pharmaceutical Alliance). This negotiation process was started a while ago and pCPA and Vertex Pharmaceuticals Inc have come to an agreement about the price of Kalydeco and Orkambi (2 other CFTR modulators), so it is hoped that this agreement will be expanded to also cover the price of Trikafta™.
Finally, once a price has been agreed upon, the individual provinces must decide if they are going to include the medication on the formulary for their province. This process can take 6-12 months.
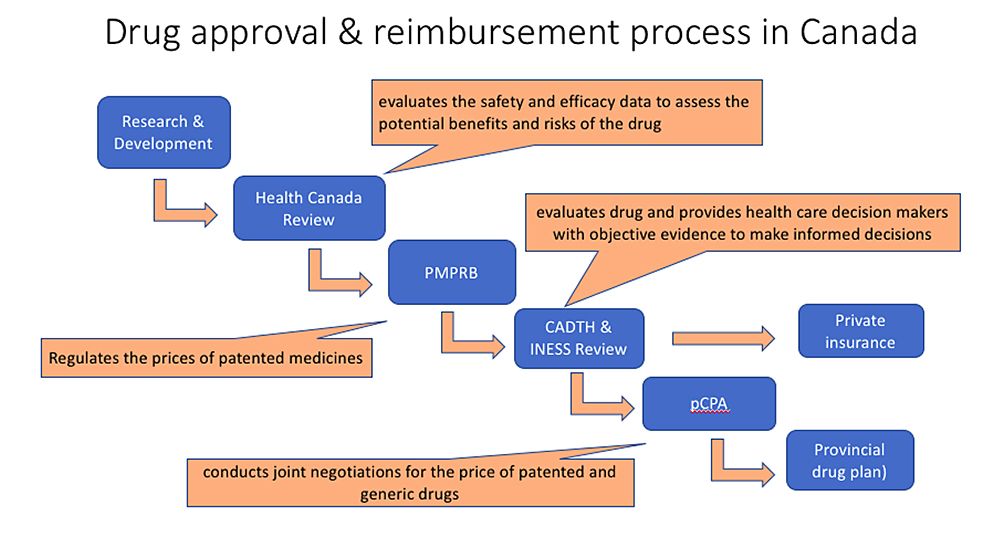
How do people get medications covered through the provincial drug plans?
In Ontario, the provincial drug plan is called the Ontario Drug Benefit (ODB) program.
To qualify for ODB you must by a citizen in Ontario and fit in any of the following categories:
- be 65 years of age or older
- 24 years of age or younger and not covered by a private insurance plan (OHIP Plus)
- receiving professional home services (Home Care)
- receiving benefits from Ontario Works or Ontario Disability Support Program
- enrolled in the Trillium Drug Program.
For more details about how to access the Ontario Drug Benefit Program, see https://www.ontario.ca/page/check-medication-coverage/.
What is the Toronto Adult CF Clinic doing to help eligible patients get access to Trikafta?
- The Toronto Adult CF Clinic uses the Canadian CF Registry to identify all of our patients with CF that have at least 1 copy of the F508del mutation and thus would be eligible for Trikaftatherapy.
- We will receive direct updates about which insurance plans have agreed to cover Trikafta.
- We will be enrolling patients in the patient assistance program (Village).
- We are increasing our staffing of pharmacists and drug access navigators to help in the enrollment process.
- We will be completing the paperwork needed by private insurers (third party payers) to approve coverage (i.e. prior authorization forms).
- We will be completing the paperwork needed by the government to approve coverage (i.e. Exceptional Access Program).We will set up sweat testing facilities at St Michael’s Hospital (sweat tests needed prior to starting therapy and for follow up).
There are a lot of people with CF eligible for Trikafta and access to therapy is a time-consuming process. How will the CF clinic prioritize the order in which patients are enrolled?
Access to Trikafta will not occur all at once. People with CF on private plans will have access sooner than patients who have their medications covered through government plans. Although this is not the way we would choose to prioritize access, it is unfortunately the reality.
We will identify the people with CF eligible for Trikafta who are on private plans and will first enrol those people who have coverage through insurance companies that have already agreed to cover Trikafta. Within this group, we will enrol based on medical need – ie “sickest” people first using lung function as a measure of disease severity.
We will then move on to all people on private insurance, even if it is not clear that their insurance will cover Trikafta. Again, the priority will be by medical need.
Finally, we will move to those people with CF who do not have private insurance and who are waiting for the decision regarding government coverage. Again, the priority will be by medical need.
What can people with CF do to help this process?
At this point in time, we are waiting for the decision from CADTH and the decision on price of Trikafta™ as negotiated between pCPA and Vertex Pharmaceuticals.
If you want to advocate for Trikafta, you can contact CF Canada, CF Get Loud and CF Treatment Society who have been advocating for Trikafta access for Canadians with CF.
What happens once Trikafta is covered?
Before you start on Trikafta, testing will be done to determine your baseline measurements of sweat chloride, lung function, blood tests of liver function, degree of lung disease (chest CT scan) and baseline quality of life. The CF clinic will schedule these tests for you.
Follow up testing will be done after being on Trikafta to demonstrate the beneficial effects in order to maintain coverage of Trikafta and to monitor for adverse side effects.
The studies of Trikafta showed dramatic improvements in measurements of lung function, rate of lung infection and symptoms. The degree of benefit is greater than the improvements seen with other CF medications.
People with two copies of the deltaF508 mutation had a 10% increase in lung function compared to treatment with the modulator tezacaftor/ivacaftor (Symdeko).
People with one copy of deltaF508 had a 14% increase in lung function compared to placebo.
People on Trikafta had a 63% reduction in the number of lung infections compared to people on placebo.
People in the studies had a significant improvement in their lung symptoms as measured by a standardized symptom score (CFQ-R).
People on Trikafta had a reduction in sweat chloride towards levels seen in people who do not have CF.
When you have received a lung transplant, your new lungs do not have CF and thus don’t have the defect in the CFTR protein. Thus, Trikafta will not have an impact on lung function after a lung transplant. Trikafta has not been tested in people with CF who have had a lung transplant so we don’t know if it would help other aspects of CF such as nutrition or sinus symptoms. Trikafta can interact with the immunosuppressive drugs needed after transplant.
Trikafta can be used in people who have had a liver transplant to help improve lung function. However, due to the interactions between Trikafta and immunosuppression medications, before starting Trikafta, discuss how you will monitor this with your transplant team.
In the clinical studies, Trikafta was shown to be safe and effective with few negative side effects over the duration of the trials.
Common early adverse effects are abdominal pain, rash, headache, diarrhea, nasal congestion and sinus symptoms and abnormalities in blood tests of liver function blood and muscle enzymes.
Certain drugs may interact with Trikafta, including some antifungal medications (itraconazole), some antibiotics (rifampin and rifabutin), some seizure medications (phenobarbital, carbamazepine, phenytoin) and some natural or herbal medications (St. John’s Wort). You should avoid foods or drinks containing grapefruit foods while on Trikafta.
Your CF pharmacist or doctor can advise you about potential interactions with Trikafta and how they can be managed so please make sure you tell your CF team about all medications and supplements you are taking before starting Trikafta.
Trikafta should not be used in people with CF who have severe liver disease. People with CF who have liver disease must be monitored very closely with bloodwork, clinical assessment and other tests (e.g. ultrasound) if they are on Trikafta. Your CF doctor can determine if it is safe for you to use Trikafta.
Trikafta may increase fertility in women with CF due to its impact on the mucus in the cervix and uterus and thus it is important to use birth control if you are on Trikafta. The studies of Trikafta were not done in women with CF who were pregnant so we do not know the effect of this drug on a developing fetus. The CF community is gathering experience about the outcomes of pregnancies that have occurred in women on Trikafta. Please talk to your CF team before considering pregnancy to get up to date information.
The studies of Trikafta were done in people with CF who were also taking all their usual CF medications (e.g. Pulmozyme, hypertonic saline, inhaled antibiotics) so we know that the addition of Trikafta to usual therapy results in these improvements in lung function.
We do not yet know if any CF therapies can be safely stopped once someone is on Trikafta. There is a study underway in the USA to see if CF medications can be safely stopped without a negative effect. Please do not stop any of your therapies without speaking to your CF team.
Follow up testing including sweat chloride, lung function, blood tests of liver function, chest CT scan and measurement of quality of life scores will be done after being on Trikafta to demonstrate the beneficial effects in order to maintain coverage of Trikafta.
Follow up visits after starting Trikafta will be done at:
- 1 month (safety bloodwork, lung function, quality of life measurement and sweat test)
- 3 months (bloodwork, lung function, quality of life measurement)
- 6 months (bloodwork, lung function, quality of life measurement)
- 9 months (bloodwork, lung function, quality of life measurement)
- 1 year (bloodwork, lung function, sweat test, quality of life measurement, CT scan)
Here are some helpful links:
https://www.Trikafta.com
https://cysticfibrosis.ca/highly-effective-modulators
You can email us at cftrmodulatorinfo@unityhealth.to
We are increasing our staffing of pharmacists and drug access navigators to help our patients in the enrollment process for Trikafta.
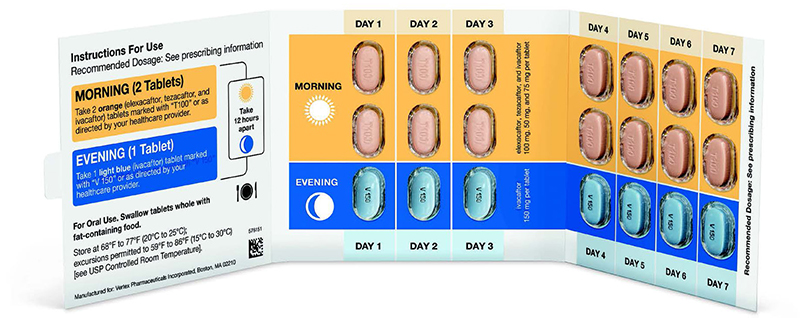
We are increasing our staffing of pharmacists and drug access navigators to help our patients in the enrollment process for Trikafta.

Access, Costs & Insurance
How will Trikafta help my CF?
The studies of Trikafta™ showed dramatic improvements in measurements of lung function, rate of lung infection and symptoms. The degree of benefit is greater than the improvements seen with other CF medications.
People with two copies of the deltaF508 mutation had a 10% increase in lung function compared to treatment with the modulator tezacaftor/ivacaftor (Symdeko).
People with one copy of deltaF508 had a 14% increase in lung function compared to placebo.
People on Trikafta™ had a 63% reduction in the number of lung infections compared to people on placebo.
People in the studies had a significant improvement in their lung symptoms as measured by a standardized symptom score (CFQ-R).
People on Trikafta™ had a reduction in sweat chloride towards levels seen in people who do not have CF.
Can I use Trikafta™ if I have had a lung transplant?
When you have received a lung transplant, your new lungs do not have CF and thus don’t have the defect in the CFTR protein. Thus, Trikafta™ will not have an impact on lung function after a lung transplant. Trikafta™ has not been tested in people with CF who have had a lung transplant so we don’t know if it would help other aspects of CF such as nutrition or sinus symptoms. Trikafta™ can interact with the immunosuppressive drugs needed after transplant.
Can I use Trikafta™ if I have had a liver transplant?
Trikafta™ can be used in people who have had a liver transplant to help improve lung function. However, due to the interactions between Trikafta™ and immunosuppression medications, before starting Trikafta™, discuss how you will monitor this with your transplant team.
What are common side effects of Trikafta™?
In the clinical studies, Trikafta™ was shown to be safe and effective with few negative side effects over the duration of the trials.
Common early adverse effects are abdominal pain, rash, headache, diarrhea, nasal congestion and sinus symptoms and abnormalities in blood tests of liver function blood and muscle enzymes.
Does Trikafta™ interact with other medications used by people with CF?
Certain drugs may interact with Trikafta™, including some antifungal medications (itraconazole), some antibiotics (rifampin and rifabutin), some seizure medications (phenobarbital, carbamazepine, phenytoin) and some natural or herbal medications (St. John’s Wort). You should avoid foods or drinks containing grapefruit foods while on Trikafta™.
Your CF pharmacist or doctor can advise you about potential interactions with Trikafta™ and how they can be managed so please make sure you tell your CF team about all medications and supplements you are taking before starting Trikafta™.
What medical conditions would prevent the use of Trikafta™?
Trikafta™ should not be used in people with CF who have severe liver disease. People with CF who have liver disease must be monitored very closely with bloodwork, clinical assessment and other tests (e.g. ultrasound) if they are on Trikafta™. Your CF doctor can determine if it is safe for you to use Trikafta™.
Can I use Trikafta™ if I am pregnant or am trying to get pregnant?
Trikafta™ may increase fertility in women with CF due to its impact on the mucus in the cervix and uterus and thus it is important to use birth control if you are on Trikafta™. The studies of Trikafta™ were not done in women with CF who were pregnant so we do not know the effect of this drug on a developing fetus. The CF community is gathering experience about the outcomes of pregnancies that have occurred in women on Trikafta™. Please talk to your CF team before considering pregnancy to get up to date information.
Can I stop using my other CF medications once I start on Trikafta™?
The studies of Trikafta™ were done in people with CF who were also taking all their usual CF medications (e.g. Pulmozyme, hypertonic saline, inhaled antibiotics) so we know that the addition of Trikafta™ to usual therapy results in these improvements in lung function.
We do not yet know if any CF therapies can be safely stopped once someone is on Trikafta™. There is a study underway in the USA to see if CF medications can be safely stopped without a negative effect. Please do not stop any of your therapies without speaking to your CF team.
How will my response to Trikafta™ be monitored?
Follow up testing including sweat chloride, lung function, blood tests of liver function, chest CT scan and measurement of quality of life scores will be done after being on Trikafta™ to demonstrate the beneficial effects in order to maintain coverage of Trikafta™.
Follow up visits after starting Trikafta™ will be done at:
- 1 month (safety bloodwork, lung function, quality of life measurement and sweat test)
- 3 months (bloodwork, lung function, quality of life measurement)
- 6 months (bloodwork, lung function, quality of life measurement)
- 9 months (bloodwork, lung function, quality of life measurement)
- 1 year (bloodwork, lung function, sweat test, quality of life measurement, CT scan)
How can I get more information about Trikafta™?
Here are some helpful links:
https://www.TrikaftaTM.com
https://www.cysticfibrosis.ca/our-programs/advocacy/access-to-medicines/TrikaftaTM
You can email us at cftrmodulatorinfo@unityhealth.to
Trikafta was approved by Health Canada on June 18, 2021. This means that the medication has received a Drug Identification Number (DIN), has met the standards of the Foods and Drugs Act and can be prescribed in Canada.
However, Trikafta now has to be produced for sale in Canada under this new DIN and thus Trikafta will not be available for sale until the end of July 2021.
After a new drug is approved by Health Canada, there are different ways that the medication can be accessed:
- Covered under private (3rd party) insurance plans. For example
- Covered under the provincial drug plans (i.e. Trillium program, ODSP, ODB) and
- Directly paid for by the individual.
The list price of Trikafta in the USA is USD $311,000 per year. We do not know what the actual cost of the medicine will be for the private and government payers.
Private insurers perform their own internal review of new medications to decide if they will add them to their list of medications that they will cover. This process takes about 1-3 months but can be longer.
Not all insurance companies will cover CFTR modulators. Even if an insurance company covers CFTR modulators, not all drug plans with that insurance company will include CFTR modulators.
Vertex has set up a patient assist program (Village) which will help assess your private insurance coverage for Trikafta.
After a discussion at your clinic visit or on the phone, you will be provided with an enrollment form for the Village patient assistance program which you will need to complete in order to access the program. As part of the enrollment process you will have to provide consent for your contact information, insurance status and some limited information about your diagnosis to be shared with the program.
Once you are enrolled, the assistance program will contact your insurance provider(s) and determine if Trikafta is covered by your plan as well as any coverage limits or additional information required from your healthcare team. The assistance program may also assess your financial situation in order to provide limited financial assistance if your insurance does not provide full coverage.
Once it is established whether you are covered for Trikafta or not, the Village patient assistance program will contact us to advise about next steps (i.e. what medical information and paperwork needs to be provided by the clinic for prior approval).
The Special Access Program was importing Trikafta into Canada when it was not Health Canada approved. Now that Health Canada has approved Trikafta, everyone on the Special Access compassionate program must be moved on to the Compassionate program with Health Canada-approved medication. We will be contacting all of those people on the Compassionate program to have them enrol in the Village Patient Assistance Program to facilitate this switch. Coverage through the Compassionate Program will continue until Trikafta is available either through the individual’s private insurance or through the provincial formulary.
After Health Canada approval, a committee called CADTH (Canadian Agency for Drugs and Technologies in Health) reviews new medications to make recommendations if they should be covered by the drug plan provided by each province. We are awaiting CADTH’s decision about Trikafta™ and this is expected in July 2021.
If CADTH recommends that Trikafta should be on the list of medications paid for by the province (i.e. the provincial formulary), then the next step is for the government to negotiate how much they will pay for Trikafta. This is done by a committee called pCPA (pan-Canadian Pharmaceutical Alliance). This negotiation process was started a while ago and pCPA and Vertex Pharmaceuticals Inc have come to an agreement about the price of Kalydeco and Orkambi (2 other CFTR modulators), so it is hoped that this agreement will be expanded to also cover the price of Trikafta.
Finally, once a price has been agreed upon, the individual provinces must decide if they are going to include the medication on the formulary for their province. This process can take 6-12 months.

In Ontario, the provincial drug plan is called the Ontario Drug Benefit (ODB) program.
To qualify for ODB you must by a citizen in Ontario and fit in any of the following categories:
- be 65 years of age or older
- 24 years of age or younger and not covered by a private insurance plan (OHIP Plus)
- receiving professional home services (Home Care)
- receiving benefits from Ontario Works or Ontario Disability Support Program
- enrolled in the Trillium Drug Program.
For more details about how to access the Ontario Drug Benefit Program, see https://www.ontario.ca/page/check-medication-coverage/.
- The Toronto Adult CF Clinic uses the Canadian CF Registry to identify all of our patients with CF that have at least 1 copy of the F508del mutation and thus would be eligible for Trikafta therapy.
- We will receive direct updates about which insurance plans have agreed to cover Trikafta.
- We will be enrolling patients in the patient assistance program (Village).
- We are increasing our staffing of pharmacists and drug access navigators to help in the enrollment process.
- We will be completing the paperwork needed by private insurers (third party payers) to approve coverage (i.e. prior authorization forms).
- We will be completing the paperwork needed by the government to approve coverage (i.e. Exceptional Access Program).We will set up sweat testing facilities at St Michael’s Hospital (sweat tests needed prior to starting therapy and for follow up).
Access to Trikafta will not occur all at once. People with CF on private plans will have access sooner than patients who have their medications covered through government plans. Although this is not the way we would choose to prioritize access, it is unfortunately the reality.
We will identify the people with CF eligible for Trikafta who are on private plans and will first enrol those people who have coverage through insurance companies that have already agreed to cover Trikafta. Within this group, we will enrol based on medical need – ie “sickest” people first using lung function as a measure of disease severity.
We will then move on to all people on private insurance, even if it is not clear that their insurance will cover Trikafta. Again, the priority will be by medical need.
Finally, we will move to those people with CF who do not have private insurance and who are waiting for the decision regarding government coverage. Again, the priority will be by medical need.
At this point in time, we are waiting for the decision from CADTH and the decision on price of Trikafta as negotiated between pCPA and Vertex Pharmaceuticals.
If you want to advocate for Trikafta, you can contact CF Canada, CF Get Loud and CF Treatment Society who have been advocating for Trikafta access for Canadians with CF.
Before you start on Trikafta, testing will be done to determine your baseline measurements of sweat chloride, lung function, blood tests of liver function, degree of lung disease (chest CT scan) and baseline quality of life. The CF clinic will schedule these tests for you.
Follow up testing will be done after being on Trikafta to demonstrate the beneficial effects in order to maintain coverage of Trikafta and to monitor for adverse side effects.

